Welcome to the ENACT Network
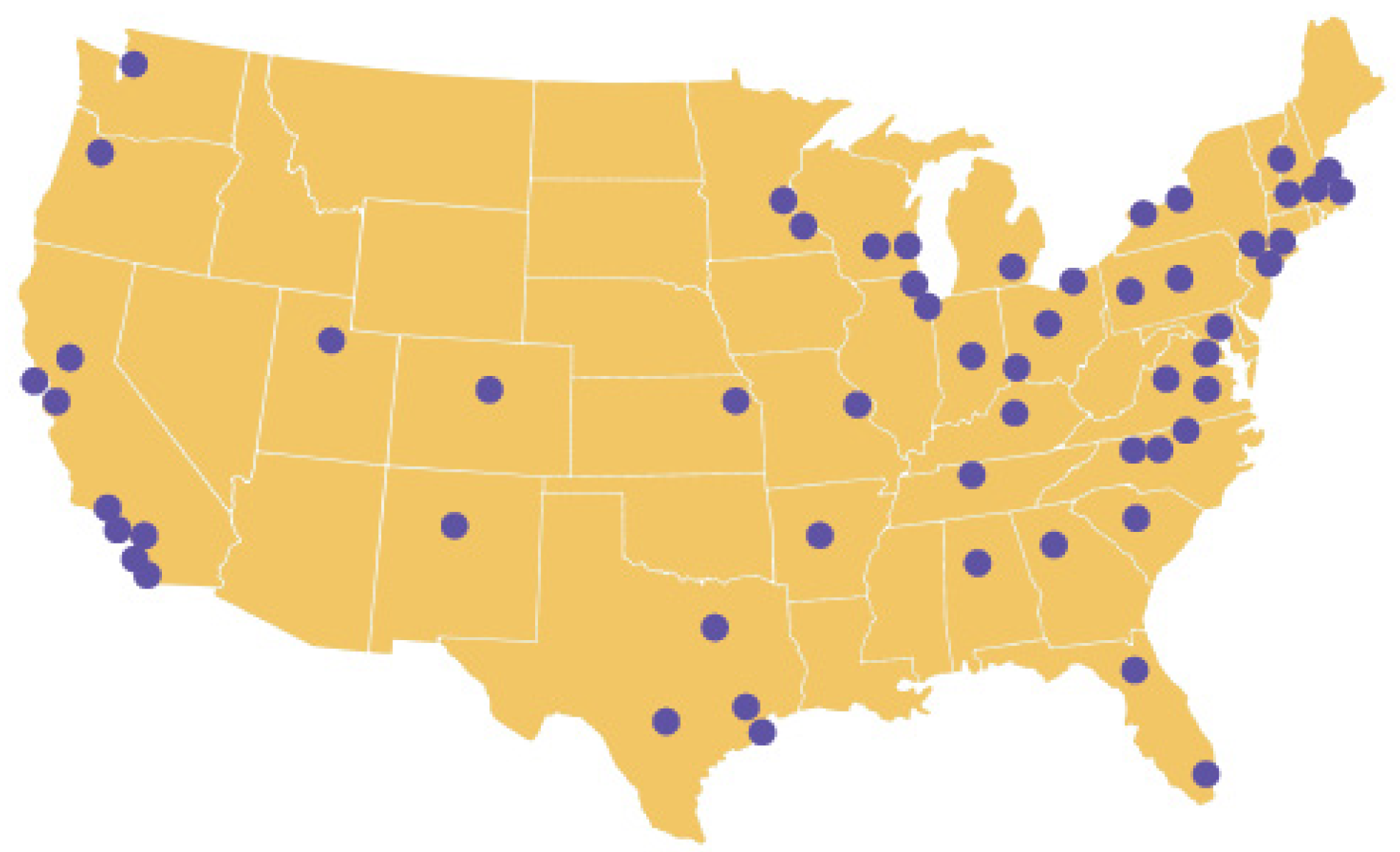
ENACT expands on the CTSA Consortium cohort discovery platform, ACT, allowing researchers and students at CTSA hubs to carry out electronic health record (EHR)-based studies on any disease or condition within a network of over 142 million patients.
This innovative platform, ENACT, emerged through collaboration among members of the National Center for Advancing Translational Sciences’ (NCATS) Clinical and Translational Science Award (CTSA) consortium. The project received financial support from the National Institutes of Health (NIH) National Center for Advancing Translational Sciences.
ENACT is available to all researchers at participating CTSA institutions.
ENACT enables…
Regulatory-Compliant EHR-Based Research
Improved data quality
Access to trainees for educational purposes
AI and machine learning analysis to large-scale EHR data sets
Informatics experts to develop and validate new tools
Clinical Decision Support
Latest ENACT News
Current capabilities of ENACT
The ENACT Network allows researchers to explore and validate feasibility for clinical studies from their desktops, providing a real-time platform for this purpose within the CTSA consortium.
It is equipped to assist researchers in designing and completing clinical studies, ensuring that the data and processes are secure and compliant with relevant regulations such as HIPAA.
Researchers can use the network to query patient data from participating sites, which includes demographics, diagnoses, lab results, and medications prescribed. This is to help determine the availability of suitable candidates for clinical studies.
Future capabilities of ENACT

ENACT History/Overview
ACT Ontology Updates: The network utilizes the ACT Ontology, which by version 4.0 included LOINC hierarchies for Social Determinants of Health (SDoH) and vital signs, additional COVID-19 terms, and RxNorm by VA class for medication classification.
SHRINE Upgrade: The Shared Health Research Information Network (SHRINE) was upgraded to version 3.1, which brought enhanced features such as demographic distributions in queries, the ability to download site counts as CSV files, and an improved user interface for managing data queries.
i2b2 Versions: The network has incorporated the i2b2 platform for data management and research facilitation, upgrading to version 1.7.12A in the fall of 2020, while also supporting version 1.7.13.
These software elements reflect the ENACT Network’s commitment to maintaining a robust, up-to-date technical infrastructure to support clinical research and data analysis.